Introduction:
Achieving undetectable measurable residual disease (uMRD) following treatment has shown significant prognostic value in several hematological malignancies. In chronic lymphocytic leukemia (CLL), uMRD is associated with improved outcomes in the era of chemoimmunotherapy (CIT). However, the role of measurable residual disease (MRD) in the context of novel agents such as Bruton tyrosine kinase inhibitors (BTKi) and/or BCL2 inhibitors (e.g. venetoclax) is uncertain. To address this question, we conducted a systematic review of the literature and performed a comprehensive meta-analysis of trials that included novel agents to evaluate the association between MRD status after treatment and PFS in patients with CLL.
Methods:
We conducted a comprehensive search of PubMed, EMBASE, Scopus, and Web of Science focusing on CLL trials reporting MRD and its association with PFS. Results were independently screened by two reviewers. Inclusion criteria included prospective phase 3 trials involving a novel agent in one of the treatment arms, while studies with incomplete information on MRD status related to PFS, reviews, abstracts, and retrospective analyses were excluded. The initial search identified 185 studies in PubMed and 402 studies in EMBASE. After applying prespecified criteria, a total of 10 phase 3 trials were included in our analysis. We extracted sample size, patient age, follow-up time, time point and tissue of MRD assessment, MRD cut-off and rate, performance status and molecular status when available. We only considered treatment arms with complete MRD information. The comparison involved patients who achieved uMRD with those exhibiting detectable MRD. When available, hazard ratios (HR) and confidence intervals (CI) were used to evaluate the relationship between MRD status and PFS. If not, we used Kaplan-Meier (KM) curves from both uMRD and MRD subgroups and extracted the coordinates points of the curves using DigitizeIt software (version 2.5.10, I. Bormann) and using the number at risk provided we estimated the HRs and CIs. A meta-analysis was performed using a random effects model from the R package metafor (Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1-48). The pooled hazard ratio estimates, and the corresponding 95% CI and prediction intervals (PI) were reported. Funnel and Baujat plots were generated to assess study heterogeneity and bias. All analysis was performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results:
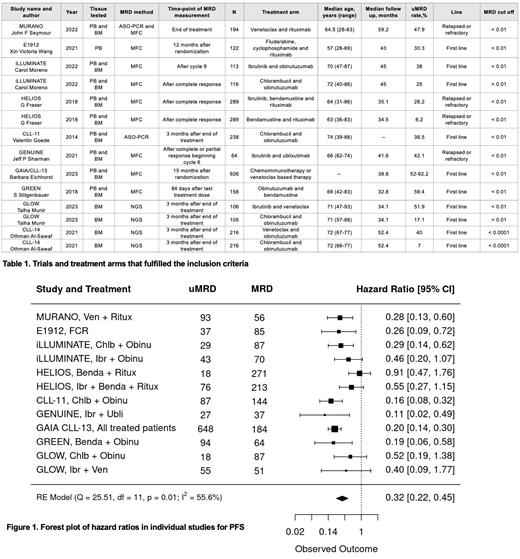
A total of 10 phase 3 trials, encompassing 14 treatment arms and involving 3152 patients, met the stringent inclusion criteria. All but one study used a uMRD cut-off of < 0.01%. Detailed information on the treatment arms and key trial characteristics is presented in Table 1. Notably, ten treatment arms were derived from first-line treatment trials, while the remaining arms pertained to the relapsed/refractory population. Only two trials (4 treatment arms) assessed MRD using next generation sequencing (NGS), while the rest used multicolor flow cytometry (MFC) (9 treatment arms) and allele-specific oligonucleotides polymerase chain reaction (ASO-PCR) (2 treatment arms). All but 3 trials assessed MRD in both bone marrow (BM) and peripheral blood (PB). One trial (2 treatment arms) was excluded from the met-analysis due to higher MRD cut-off. All studies included in the meta-analysis measured uMRD (<0.01) versus MRD. The PFS pooled HR estimate for achieving uMRD at 0.01 cut off from the meta-analysis was 0.32 (95% CI [0.22, 0.45]), with minimal bias observed on the Funnel Plot.
Conclusions:
In this comprehensive meta-analysis, comprising 9 phase 3 trials, achieving uMRD was strongly associated with PFS. These findings further establish the prognostic role of MRD in CLL management and its potential use as a surrogate marker in clinical trials. As the body of evidence on MRD in CLL continues to grow, it becomes imperative to standardize the optimal time point, methodology, and tissue for MRD assessment. Substantial heterogeneity in these aspects underscores the necessity for unified guidelines to ensure the accurate and consistent evaluation of MRD status in clinical practice.
Disclosures
Tuna Aguilar:Pfizer: Speakers Bureau; Abbvie: Speakers Bureau; Novartis: Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal